The reaction of zinc and EXCESS dilute hydrochloric acid is used to investigate factors that affect reaction rate. The balanced equation for the reaction is:

The reaction conditions used and the results obtained for each experiment are summarised in the table below.
The same mass of zinc is used in all the experiments. The zinc is completely covered in all reactions. The reaction time is the time it takes the reaction to be completed.
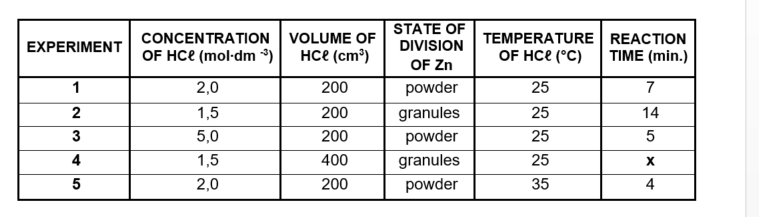
5.1 Experiment 1 and experiment 5 are compared. Write down the independent
variable. (1)
5.2 Define reaction rate. (2)
5.3 Write down the value of x in experiment 4. (2)
5.4 The Maxwell-Boltzmann energy distribution curves for particles in each of experiments 1, 3 and 5 are
shown below.

Identify the graph (A or B or C) that represents the following:
5.1.4 Experiment 3. Give a reason for the answer. (2)
5.1.5 Experiment 5. Give a reason for the answer. (2)
5.5 Experiment 6 is now conducted using a catalyst and the SAME reaction conditions as for Experiment 1.
5.5.1 What is the function of the catalyst in this experiment? (1)
5.5.2 How will the heat of reaction in experiment 6 compare to that in experiment 1? Choose from:
GREATER THAN, EQUAL TO or LESS THAN. (1)
5.6 Calculate the average rate of the reaction (in mol·min-1) with respect to zinc
for experiment 2 if 1,5 g of zinc is used. (4)
[15]
