The reaction below is used in the Haber process to manufacture ammonia
N2(g) + 3H2(g) → 2NH3(g)
The boiling points of the substances in the reaction are as follows:
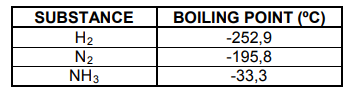
3.1 Refer to the intermolecular forces and explain the difference in boiling point
between NH3 and N2. (3)
3.2 Write down the FORMULA of the substance in the table that will have the
lowest melting point. (1)
3.3 Explain why H2 will evaporate faster than N2. Refer to the type and relative
strength of the intermolecular forces. (3)
3.4 Write down the FORMULA of the substance in the table that will have the
highest vapour pressure. Explain your answer. (3)
[10]
