Hydrogen cyanide (HCN) is a very poisonous compound used in the manufacturing of
plastics, mining of gold and as a poison.
2.1 Define the term chemical bond. (2)
2.2 Draw Lewis structures for:
2.2.1 HCN (2)
2.2.2 H2O (2)
2.3 What is the shape of the HCN molecule? (1)
2.4 Calculate the electronegativity difference for the CN bond. (1)
2.5 What is polarity of the HCN molecule? Write only POLAR or NON-POLAR. (1)
The table below indicates the values of the bond length and bond energy of the
different bonds in HCN.
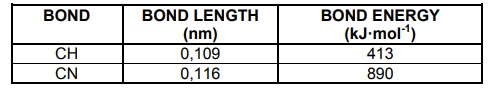
2.6 Explain why the bond energy of the CN bond is more than the bond energy of
the CH bond. (2)
2.7 Explain the difference between the bond length of the CH bond and the bond
length of the CN bond. (2)
2.8 Will HCN be soluble in water? Write only YES or NO. (1)
2.9 Explain the answer to QUESTION 2.8 by referring to the polarity and
intermolecular forces of the compounds. (3)
[17]
