A group of learners prepare a 0,25 mol∙dm-3 solution of sodium carbonate by dissolving
a 14,2 g sample of hydrated sodium carbonate (Na2CO3∙xH2O) in 200 cm3 of water.
7.1 Explain the meaning of the term hydrated. (1)
7.2 Write down a BALANCED CHEMICAL EQUATION to show how sodium
carbonate dissociates in water. (2)
7.3 Learners then take 10 cm3 of the prepared solution and allow it to react
completely with 5 cm3 of dilute hydrochloric acid, according the following
balanced chemical equation:
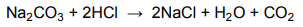
7.3.1 Define the term a mole of a substance. (2)
7.3.2 What type of chemical reaction is represented by the chemical
equation above? (1)
7.3.3 Calculate the number of moles of hydrochloric acid in 5 cm3 of
hydrochloric acid if its concentration is 1 mol∙dm-3. (3)
7.4 Calculate the mass of sodium chloride formed in the reaction in
QUESTION 7.3. (5)
[14]
