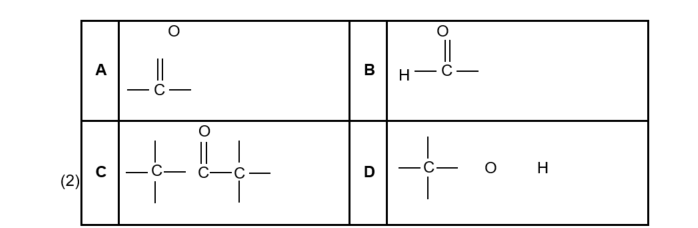
1.1 Which ONE of the following is the structural formula of the functional group of the KETONES?
1.2 Which ONE of the formulae below represents an ALKANE?
A C2H4
B C5H10
C C14H30
D C8H14 (2)
1.3 Consider the organic compound below.
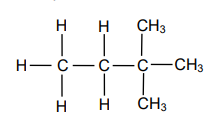
The IUPAC name of this compound is …
A 2,3-dimethyl butane.
B 3,3-dimethyl butane.
C 2,2-dimethyl butane.
D 1,1,1-trimethyl propane. (2)
1.4 Activation energy can best be described as the minimum energy required to …
A cause effective collisions.
B make reactant molecules collide.
C change the orientation of reactant molecules.
D increase the kinetic energy of reactant molecules. (2)
1.5 Which statement is CORRECT for a system in DYNAMIC EQUILIBRIUM?
A All reactants are used up.
B The forward reaction is equal to the reverse reaction.
C All substances in the reaction are of equal concentration.
D The concentration of the reactants and products remain constant. (2)
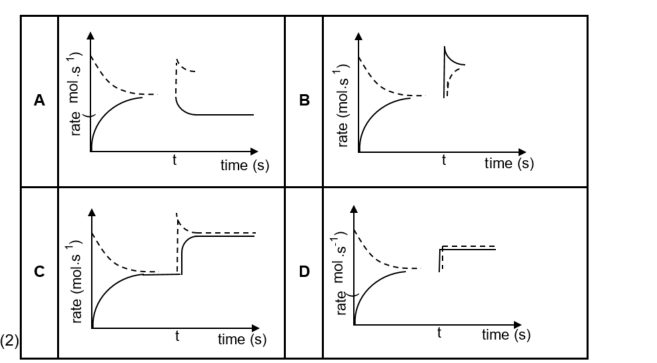
1.6 Initially, a certain amount of P(g) was placed in an empty container. The hypothetical reaction reaches
equilibrium in a closed container according to the following balanced equation:

At time t, the temperature is increased.
Which graph below best illustrates the resulting changes in the rates of the forward and reverse reactions after the temperature is increased?
1.7 Reactions I and II below have equilibrium constants (Kc) greater than 1.
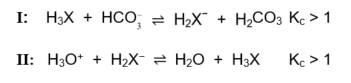
Based on the reactions above, the ACIDS in order of INCREASING STRENGTH (weakest to strongest) are …
A H3X, H2X−, H3O+
B H2CO3, H3X, H3O+
C H3X, H2CO3, H3O+
D H3X, H3O+, H2CO3 (2)
1.8 Consider the cell notation for a galvanic cell below.

Which ONE of the following half-reactions takes place at the ANODE of this cell?
A 2H+(aq) + 2e−→ H2(g)
B H2(g) → 2H+(aq) + 2e−
C Ni2+(aq) + 2e−→ Ni(s)
D Ni(s) → Ni2+(aq) + 2e− (2)
1.9 Which ONE of the following is applicable to an ELECTROLYTIC CELL?
A Reduction takes place at the anode.
B Oxidation takes place at the cathode.
C It uses alternating current.
D A battery is used for the cell to function. (2)
1.10 The flow diagram below shows four stages (A, B, C and D) in the conversion of sulphur to sulphuric acid.
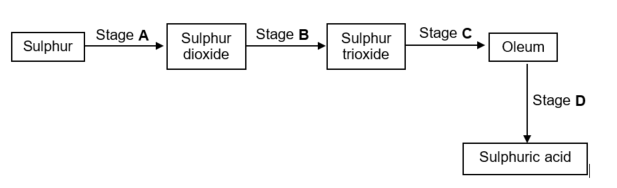
At what stage is a catalyst used?
A A
B B
C C
D D (2)
[20]
