Two test tubes, A and B, both contain a sodium salt solution. After a small amount of
barium chloride is added, the solution in both test tubes forms a white precipitate.
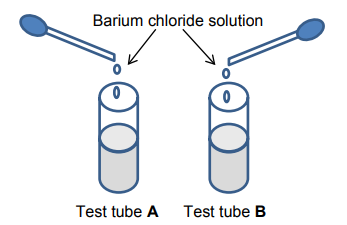
9.1 Write down the type of reaction that takes place in the test tubes. (1)
9.2 A concentrated nitric acid solution is then added into each test tube to
establish which one contains carbonate ions and which one contains sulphate
ions.
Bubbles are formed in test tube A. There is no reaction in test tube B.
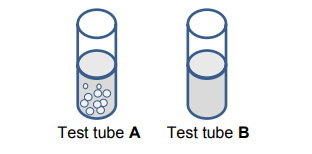
9.2.1 Identify which IONS (CARBONATE or SULPHATE) are present in
test tube B. (1)
9.2.2 Write down a BALANCED CHEMICAL EQUATION that represents
the reaction between nitric acid and the precipitate formed in test
tube A. (3)
9.3 A solution of sodium carbonate was prepared by dissolving 5 g of the powder
in 100 cm3 of water. The solution reacted with a barium chloride solution,
according to the following balanced chemical equation:
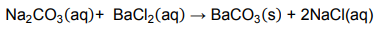
9.3.1 Calculate the mass of barium carbonate that should form in this
reaction. (5)
It was found that only 8,3 g precipitate formed.
9.3.2 Calculate the percentage yield. (2)
[12]
