8.1 Corrosion is a redox reaction that takes place in the presence of oxygen and water. Rusting is the corrosion of iron leading to the formation of iron(III) ions.
8.1.1 Define oxidation in terms of electron transfer. (2)
A cleaned copper rod and a cleaned iron nail are placed in a beaker containing water at 25°C, as shown below.
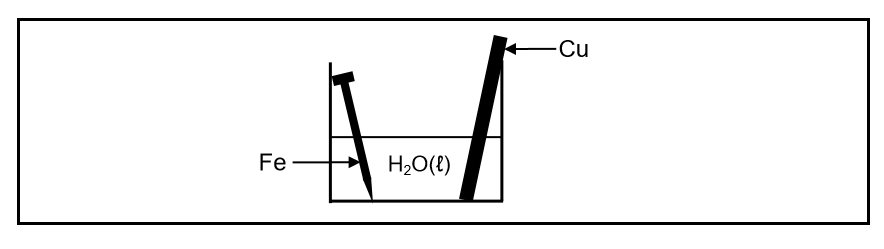
After a while it was observed that the iron nail was coated with rust. The copper rod showed no visible signs of corrosion.
8.1.2 Write down the half-reaction for the iron nail. (2)
8.1.3 Does iron act as REDUCING AGENT or OXIDISING AGENT in the beaker? (1)
8.1.4 Explain the above observation by referring to the Table of Standard Reduction Potentials. (3)
To prevent rusting of an underground iron pipe, the pipe is connected to a metal (Q) that corrodes easily.
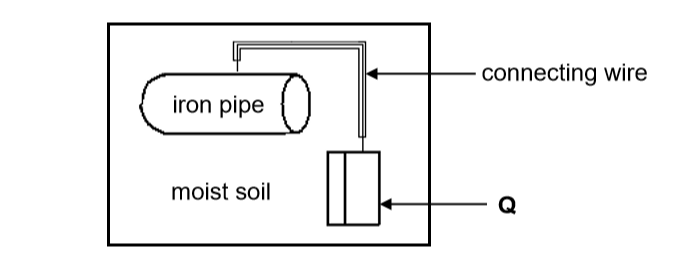
8.1.5 You are given two metals, Zn and Cu, to use as metal Q. Which metal would more suitable? Give a reason. (2)
8.2 A galvanic cell is constructed using a Fe | Fe3+ half-cell and a Cu | Cu2+ half-cell.
8.2.1 Write down the overall (net) cell reaction that takes place when the cell is functioning. (3)
8.2.2 Calculate the cell potential of this cell under standard conditions. (4)
[17]
