7.1 Ammonium chloride crystals, NH4Cl(s), dissolve in water to form ammonium and chloride ions. The ammonium ions react with water according to the balanced equation below:
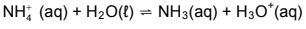
7.1.1 Write down the name of the process described by the underlined sentence. (1)
7.1.2 Is ammonium chloride ACIDIC or BASIC in aqueous solution? Give a reason for the answer. (2)
7.2 A certain fertiliser consists of 92% ammonium chloride. A sample of mass x g of this fertiliser is dissolved in 100 cm3 of a 0,10 mol∙dm-3 sodium hydroxide solution, NaOH(aq). The NaOH is in excess.
The balanced equation for the reaction is:

7.2.1 Calculate the number of moles of sodium hydroxide in which the sample is dissolved. (3)
During a titration, 25 cm3 of the excess sodium hydroxide solution is titrated with a 0,11 mol∙dm-3 hydrochloric acid solution, HCl(aq). At the endpoint it is found that 14,55 cm3 of the hydrochloric acid was used to neutralise the sodium hydroxide solution according to the following balanced equation:
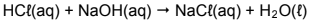
7.2.2 Calculate the mass x (in grams) of the fertiliser sample used. (8)
7.3 Calculate the pH of a 0,5 moldm-3 sodium hydroxide solution at 25 °C. (4)
[18]
