The following reaction between ammonia and oxygen takes place in a closed system
at constant pressure and temperature:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
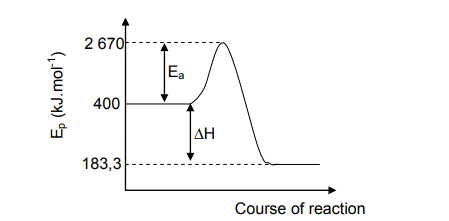
7.1 Define the term activation energy. (2)
7.2 Give a reason why this reaction is exothermic. (1)
7.3 Calculate the heat of reaction. (3)
7.4 Redraw the graph and indicate with a dotted line the effect of a catalyst on the
activation energy. (2)
7.5 State Avogadro’s law in words. (2)
7.6 If 6 dm3 of NH3 and 9 dm3 of O2 are used, calculate the TOTAL VOLUME of
the gases at the end of the reaction. (4)
7.7 The reaction above is the first step in the manufacturing of an acid. This acid
contains 1,59% hydrogen, 22,2% nitrogen and 76,2% oxygen. Determine the
empirical formula of the acid. (5)
[19]
