Study the table below and answer the questions that follow.
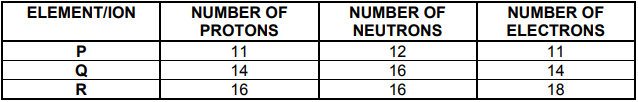
4.1 Define the term atomic number. (2)
4.2 Write down the:
4.2.1 Chemical symbol of element Q using the notation ![]() (2)
(2)
4.2.2 Element (P, Q or R) that is an alkali metal (1)
4.2.3 Chemical symbol of R (2)
4.3 Element P reacts with oxygen to form the compound with the chemical
formula P2O.
4.3.1 Predict the chemical formula that element Rb in the periodic table
will form when it reacts with oxygen. (2)
4.3.2 Explain the answer to QUESTION 4.3.1. (2)
4.4 What is the trend in ionisation energy as you move from element P to element R? Write down only INCREASES, DECREASES or REMAINS THE SAME. Explain the answer. (4)
4.5 How many electrons does an ION of element P have? Draw the Aufbau
diagram of this ion. (3)
4.6 When orbitals of identical energy are available, electrons are placed in
individual orbitals before they are paired. Give the name of this rule. (1)
4.7 Element Y occurs as these isotopes in the following proportions:
Y – 28(92,23%); Y – 29(4,68%); Y – 30(3,09%)
Calculate the relative atomic mass of element Y. (3)
[22]
