11.1 A teacher in a science class explains how different types of spectra are obtained. The teacher uses the simplified diagrams shown below for the explanation.
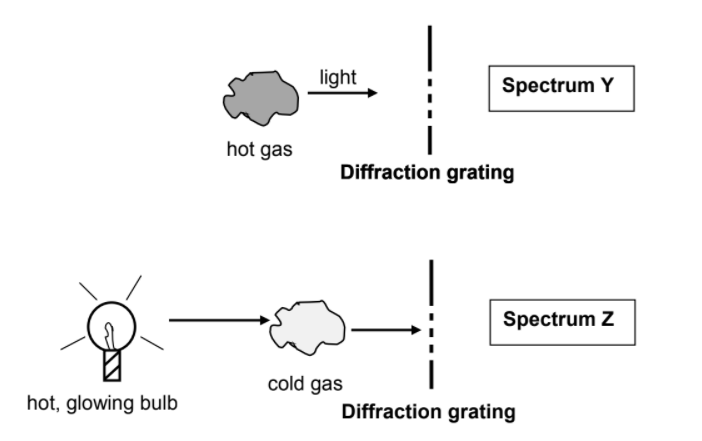
Name the type of spectrum of:
11.1.1 Y (1)
11.1.2 Z (1)
11.2 In an excited atom, electrons can ‘jump’ from lower energy levels to higher energy levels. They can also ‘drop’ from higher energy levels to lower energy levels.
The diagram below (not drawn to scale) shows some of the transitions for electrons in an excited atom.
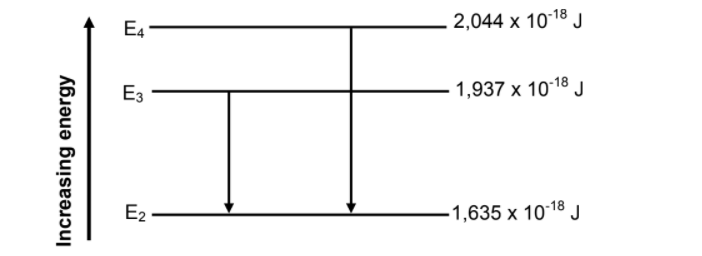
11.2.1 Do the transitions indicated in the diagram lead to ABSORPTION or EMISSION spectra? (1)
11.2.2 Calculate the frequency of the photon produced when an electron in an excited atom makes a transition from E4 to E2, as shown in the diagram. (4)
The threshold frequency of a metal, Q, is 4,4 x 1014 Hz.
11.2.3 Calculate the kinetic energy of the most energetic electron ejected when the photon produced in QUESTION 11.2.2 is incident on the surface of metal Q. (4)
Another metal, R, has a threshold frequency of 7,5 x 1014 Hz.
11.2.4 Will the photon produced in QUESTION 11.2.2 be able to eject electrons from the surface of metal R? Write down only YES or NO.
Give a reason for the answer. (2)
[13]
TOTAL: 150
