In the flow diagram below, I and II represent industrial processes used in the fertiliser industry.
P and Q are chemical reactions that take place to produce ammonium sulphate and fertiliser Y respectively.
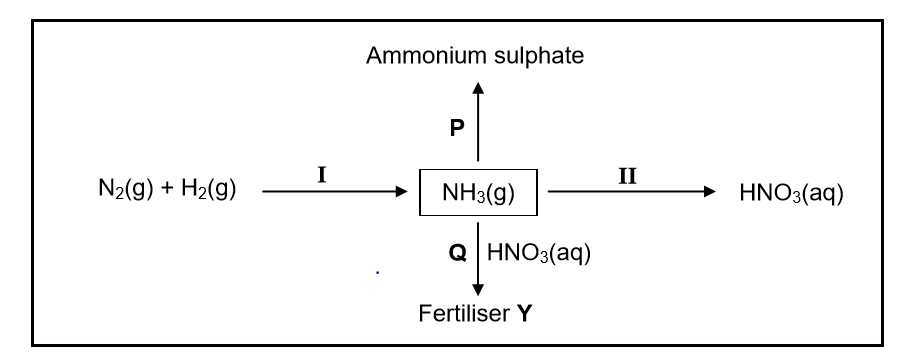
10.1 Write down the name of the industrial process:
10.1.1 I (1)
10.1.2 II (1)
10.2 Write down the NAME or FORMULA of:
10.2.1 Fertiliser Y (1)
10.2.2 The catalyst used in process I (1)
10.3 In reaction P, NH3(g) reacts with another substance. Write down a balanced equation for this reaction. (3)
10.4 The following substances are present in a bag of fertiliser:
- 20 kg ammonium nitrate (NH4NO3)
- 12 kg sodium phosphate (Na3PO4)
- 18 kg potassium chloride (KCℓ)
Calculate the NPK ratio of the fertiliser. (5)
[12]
TOTAL: 150
