The vapour pressure versus temperature graph below was obtained for four unknown
liquids (A, B, C and D). Atmospheric pressure is measured as 101,3 kPa.
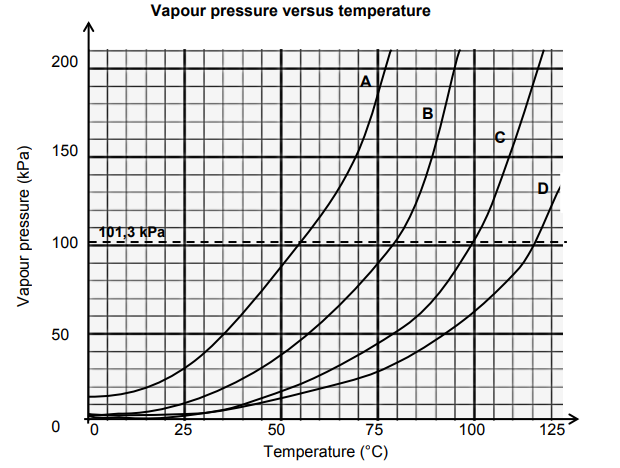
3.1 Define the term boiling point. (2)
Use the information given in the graph to answer the questions that follow.
3.2 Write down the:
3.2.1 Boiling point of liquid B (1)
3.2.2 Liquid which remains a liquid at 115 °C (1)
3.2.3 Liquid that is most likely to be water (1)
3.3 State the PHASE CHANGE that takes place at the stage when the vapour
pressure is equal to atmospheric pressure. (1)
3.4 What happens to the temperature of a liquid during a phase change? Write
down only INCREASES, DECREASES or REMAINS THE SAME. (1)
3.5 Explain the answer to QUESTION 3.4. (2)
3.6 Which liquid (A, B, C or D) has the weakest intermolecular forces? Give a
reason for the answer. (3)
3.7 What is the relationship between vapour pressure of the liquid and
temperature? (2)
[14]
