The boiling points of different organic compounds are given below.
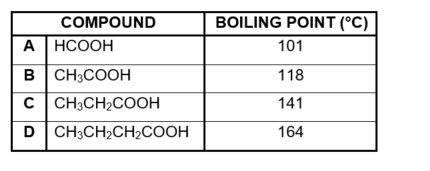
3.1 Define boiling point. (2)
3.2 Write down the:
3.2.1 Name of the FUNCTIONAL GROUP of these compounds (1)
3.2.2 IUPAC name of compound C (1)
3.2.3 Structural formula of the FUNCTIONAL isomer of compound B (2)
3.3 Which ONE of the compounds, A or B or C, has the highest vapour pressure? Refer to the data in the table to give a reason for the answer. (2)
3.4 The boiling point of compound B is now compared with of compound X.
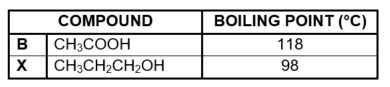
3.4.1 Besides the conditions used to determine boiling points, give a reason why this is a fair
comparison. (1)
3.4.2 Is compound X a PRIMARY, SECONDARY or TERTIARY alcohol?
Give a reason for the answer. (2)
3.4.3 Fully explain the difference between the boiling points by referring to the types of intermolecular forces present in each of these compounds. (4)
[15]
