The simplified diagram below shows a blast furnace used for the extraction of iron from iron ore. P represents a reactant added to the blast furnace. Q and R represent products that leave the blast furnace.
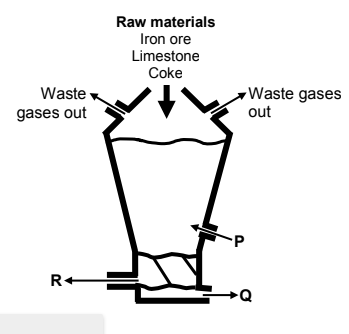
Limestone and coke are added to the blast furnace, as shown in the diagram above. Write down the function of:
10.1.1 Limestone (1)
10.1.2 Coke (1)
10.2 Write down the NAME of the substance represented by:
10.2.1 P (1)
10.2.2 Q (1)
10.2.3 R (1)
10.3 Write down the NAME or FORMULA of ONE waste gas formed during the extraction of iron from iron ore. (1)
10.4 The balanced equation for the extraction of iron from iron ore is: Fe2O3(s) + 3CO(g) ⭢2Fe(s) + 3CO2(g)
Write down the:
10.4.1 Function of carbon monoxide in this reaction (1)
10.4.2 FORMULA of the substance that is reduced (1)
[8]
TOTAL: 150
