When sulphuric acid reacts with water, it ionises in two steps, as shown in the two balanced equations below.
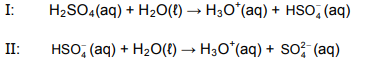
8.1 Define an acid in terms of the Lowry-Brønsted theory. (2)
8.2 Write down the FORMULA of:
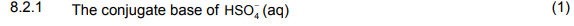

8.2.3 A substance that acts as ampholyte in these reactions (1)
8.3 A few drops of bromothymol blue indicator are added to a potassium hydroxide solution in a beaker. A dilute sulphuric acid solution is now gradually added to this solution until the colour of the indicator changes.
Write down the:
8.3.1 Type of reaction that takes place (Write down only REDOX, PRECIPITATION or NEUTRALISATION.) (1)
8.3.2 Balanced equation for the reaction that takes place (3)
8.3.3 Colour change of the indicator (2)
8.3.4 NAME of the salt formed in this reaction (1)
[12]
