Electronegativity of atoms may be used to explain the polarity of bonds.
2.1 Define the term electronegativity. (2)
2.2 Draw the Lewis diagram of an oxygen difluoride molecule. (2)
2.3 Calculate the electronegativity difference between O and F in oxygen
difluoride and predict the polarity of the bond. (2)
2.4 A polar bond does not always lead to a polar molecule.
Explain the statement by referring to OF2 and CO2 molecules. In your
explanation, include the polarity of the bonds and the shape of the molecules. (4)
2.5 The diagram below shows the energy change that takes place when two
atoms move towards each other.
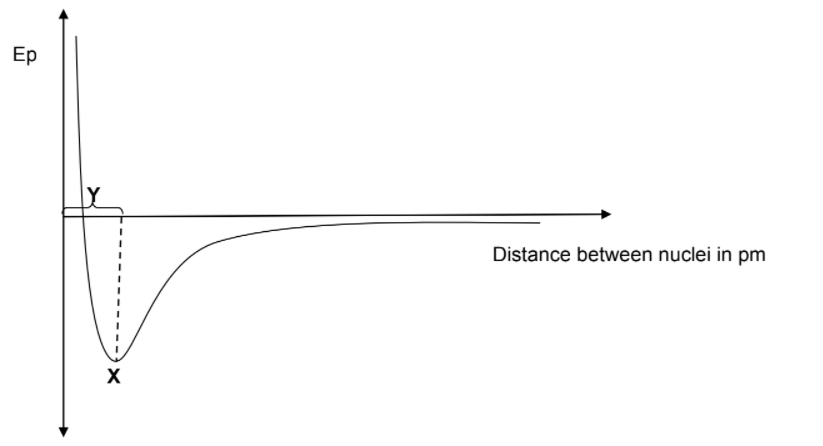
2.5.1 What does X and Y represent? (2)
2.5.2 Define the concept represented by X. (2)
2.5.3 Explain the relationship between bond order, bond length and bond
energy. (3)
[17]
