Various options are provided as possible answers to the following questions. Each
question has only ONE correct answer. Choose the answer and write only the
letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for
example 1.11 E.
1.1 Which ONE of the bonds between the atoms below has the highest polarity?
A H – C
B H – Cℓ
C H – O
D H – N
(2)
1.2 Solid iodine sublimes easily. The intermolecular forces present in iodine
are …
A London forces.
B hydrogen bonding.
C ion-dipole forces.
D dipole-dipole forces.
(2)
1.3 The graph below shows how the potential energy varies with distance
between the nuclei of two nitrogen atoms when a double bond between the
nitrogen atoms (N = N) is formed.
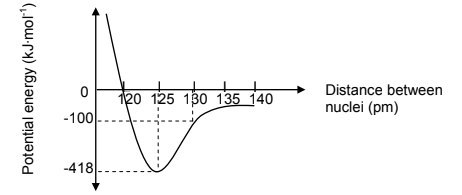
Choose from the table the bond length and bond energy for N = N.
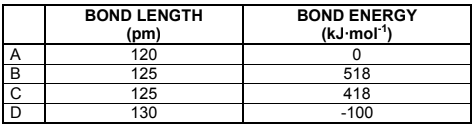
(2)
1.4 According to Boyle’s law, …

(2)
1.5 One mole of any gas occupies the same volume at the same temperature and
pressure.
This statement is known as …
A Charles’s law.
B Gay Lussac’s law.
C Avogadro’s law.
D the ideal gas LAW.
(2)
1.6 One mole of a gas, SEALED in a container, has volume V at temperature T
and pressure p. If the pressure is increased to 3p, the ratio between the
volume and temperature (V : T) is …
A 1 : 1⁄3
B 3 : 1
C 1⁄3 : 3
D 1 : 3
(2)
1.7 The chemical equation that represents an endothermic reaction:
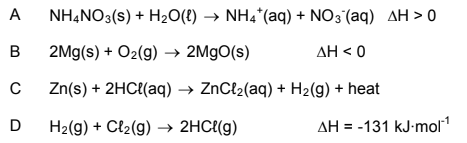
(2)
1.8 The CORRECT formula for nitric acid:
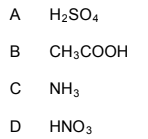
(2)
1.9 Consider the reaction below.
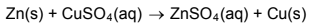
Which substance is the oxidising agent?
(2)
1.10 Which ONE of the reactions below will produce the salt sodium ethanoate
(sodium acetate)?
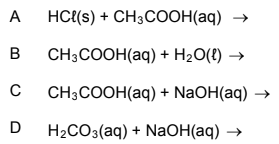
(2)
[20]
