In the electrochemical cell below, carbon electrodes are used during the electrolysis of a concentrated sodium chloride solution.
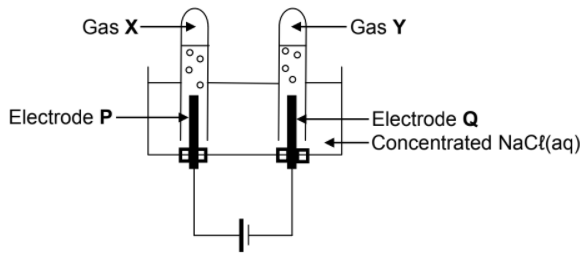
The balanced equation for the net (overall) cell reaction is:
2H2O(ℓ) + 2Cℓ ─(aq) → Cℓ2(g) + H2(g) + 2OH─(aq)
9.1 Is the reaction EXOTHERMIC or ENDOTHERMIC? (1)
9.2 Is electrode P the ANODE or the CATHODE? Give a reason for the answer. (2)
9.3 Write down the:
9.3.1 NAME or FORMULA of gas X (1)
9.3.2 NAME or FORMULA of gas Y (1)
9.3.3 Reduction half-reaction (2)
9.4 Is the solution in the cell ACIDIC or ALKALINE (BASIC) after completion of the reaction? Give a reason for the answer. (2)
[9]
