6.1 The decomposition of hydrogen peroxide in the presence of a catalyst at standard pressure and room temperature is given by the unbalanced chemical equation below.

The oxygen gas is collected and the volume is recorded over a period of time.
The reaction is completed at time t.
The results are plotted on a graph of volume O2 versus time, as shown
below.
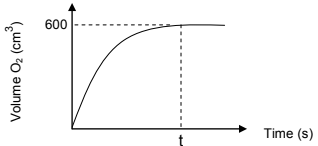
Take the molar gas volume (Vm) as 24,45 dm3 at room temperature and
standard pressure.
6.1.1 Balance the equation. (2)
6.1.2 How would a catalyst affect the reaction?(2)
6.1.3 Use the information on the graph to calculate the mass of hydrogen
peroxide that decomposed.(6)
6.2 In an experiment, a learner adds 500 cm3 hydrochloric acid (HCℓ), with a
concentration of 0,36 mol·dm-3, to 1,2 g of magnesium in a test tube. She
records the change in the mass of magnesium as the reaction proceeds at
regular intervals. The balanced chemical equation for the reaction is:
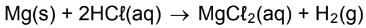
The change in the mass of magnesium during the reaction is shown on the
graph below.

6.2.1 Identify the limiting agent in this reaction. Give a reason for the
answer.(2)
6.2.2 Calculate the number of moles of unreacted hydrochloric acid in
the test tube after 3 minutes.(7)
[19]
