QUESTION 5 (Start on a new page.)
The calcium carbonate (CaCO3) in antacid tablets reacts with dilute hydrochloric acid
(HCℓ) according to the following balanced equation:
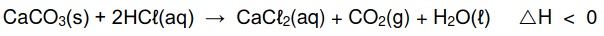
5.1 Is the above reaction EXOTHERMIC or ENDOTHERMIC? Give a reason for
the answer. (2)
An antacid tablet of mass 2 g is placed in HCℓ(aq). After 30 s the mass of the tablet
was found to be 0,25 g.
5.2 Calculate the average rate (in g∙s-1) of the above reaction. (3)
The antacid tablet contains 40% calcium carbonate. Another antacid tablet of mass 2 g
is allowed to react completely with HCℓ(aq).
5.3 Calculate the volume of carbon dioxide, CO2(g) that will be collected at STP.
Assume that all the CO2(g) produced is from the calcium carbonate. (5)
The reaction rate of similar antacid tablets with excess HCℓ(aq) of concentration
0,1 mol∙dm-3 at DIFFERENT TEMPERATURES is measured. The graph below was
obtained.
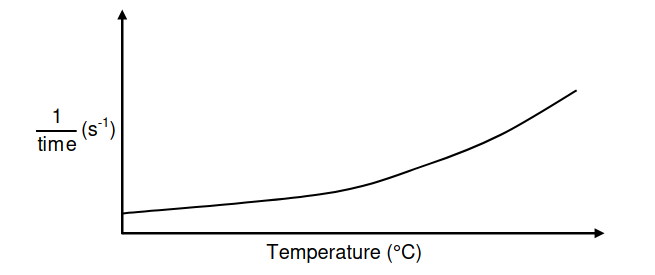
Use the information in the graph to answer the following questions.
5.4 Write down ONE controlled variable for this investigation. (1)
5.5 Write down a conclusion that can be made from the graph. (2)
5.6 Use the collision theory to fully explain the answer to QUESTION 5.5 (3)
5.7 Redraw the graph above in the ANSWER BOOK. On the same set of axes,
sketch the curve that will be obtained if HCℓ(aq) of concentration 0,2 mol∙dm-3
is now used. Label this curve Y. (2)
[18]
