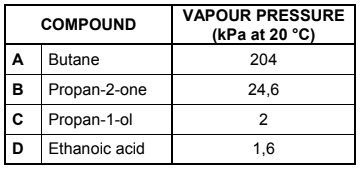
Four compounds of comparable molecular mass are used to investigate the effect of functional groups on vapour pressure. The results obtained are shown in the table below.
4.1 Define the term functional group of an organic compound. (2)
4.2 Which ONE of the compounds (A, B, C or D) in the table has the:
4.2.1 Highest boiling point (Refer to the vapour pressures in the table to give a reason for the
answer.) (2)
4.2.2 Weakest intermolecular forces (1)
4.3 Refer to the type of intermolecular forces to explain the difference between the vapour pressure of compound A and compound B. (3)
4.4 The vapour pressures of compounds C and D are much lower than those of compounds A and B. Name the type of intermolecular force in A and B that is responsible for this difference. (1)
4.5 Briefly explain the difference in vapour pressure between compound C and compound D. (2)
4.6 During a combustion reaction in a closed container of adjustable volume, 8 cm3 of compound A (butane) reacts in excess oxygen according to the following balanced equation:
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g)
If the initial volume of the oxygen in the container was 60 cm3 , calculate the TOTAL volume of the gases that are present in the container at the end of the reaction. All the gases in the container are at the same temperature and pressure. (5)
[16]
