A learner investigates the relationship between the pressure and volume of an enclosed DIATOMIC gas at 25°C. He records the volume of the gas for different pressures in the table below.
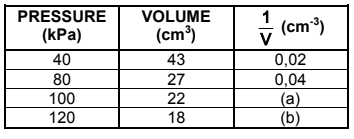
4.1 Write down the name of the gas law being investigated. (1)
Answer QUESTION 4.2 and QUESTION 4.3 on the attached ANSWER SHEET.


4.4 Use the graph to determine the volume of the gas at 68 kPa. (2)
4.5 The mass of the enclosed DIATOMIC gas is 2,49 x 10-2 g.
4.5.1 Use the conditions at a pressure of 100 kPa and calculate the molar mass of the enclosed gas. (6)
4.5.2 Write down the molecular formula of the enclosed gas. (1)
4.6 The sketch graph below shows the relationship between volume and temperature for an ideal gas.
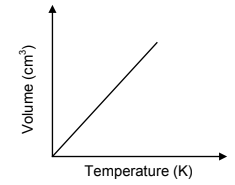
4.6.1 Redraw the above graph in the ANSWER BOOK. On the same set of axes, use a BROKEN LINE to sketch the graph that will be obtained for the diatomic gas above. (1)
4.6.2 Fully explain why this diatomic gas deviates from ideal behaviour. (3)
[19]
