Consider the following substances:
C90; NaCℓ; CO2; Fe; H2O
2.1 Write down a substance from the list above that is the following:
2.1.1 A molecular structure (1)
2.1.2 A metallic structure (1)
2.1.3 A covalent network structure (1)
2.1.4 An ionic network structure (1)
2.2 Draw the Lewis dot diagram for the CO2 molecule. (2)
2.3 Identify the type of chemical bond in H2O. (1)
2.4 Draw the Lewis dot diagrams to show the formation of NaCl. (3)
2.5 Study the models of compounds A, B and C below and answer the questions
that follow.
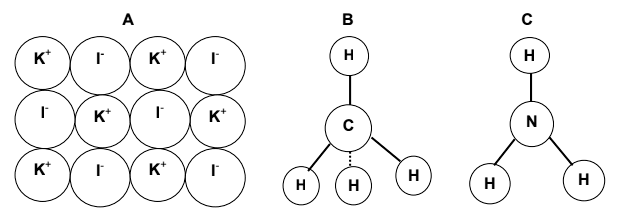
Write down the:
2.5.1 Chemical name of compound A (1)
2.5.2 Chemical formula of compound B (1)
2.5.3 Common name of compound C (1)
2.6 Many of the gases in air are very useful. An important industrial process,
fractional distillation of liquid air, separates these gases from one another.
Consider the diagram below and answer the questions that follow.
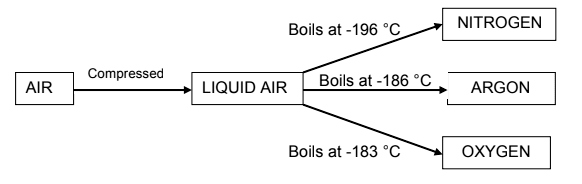
2.6.1 Is this separation process physical or chemical? (1)
2.6.2 Which physical property is used to separate the gases after they
have been liquefied? (1)
2.6.3 Which gas has the weakest intermolecular forces? Explain the
answer. (2)
2.7 State how EACH of the following changes when liquid nitrogen changes into
gaseous nitrogen. Write down only INCREASE, DECREASE or REMAIN THE
SAME.
2.7.1 Spaces between the particles (1)
2.7.2 Strength of the forces between the particles (1)
2.7.3 Energy of the particles (1)
[20]
