The letters A to F in the table below represent six organic compounds.
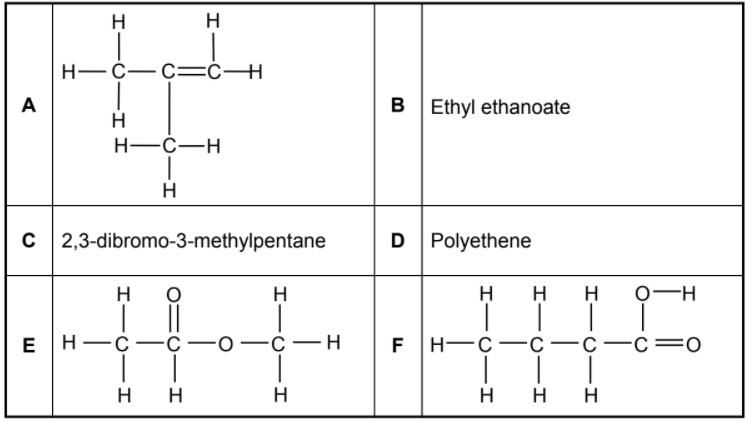
2.1 Write down the LETTER that represents the following:
2.1.1 A hydrocarbon (1)
2.1.2 A functional isomer of compound F (1)
2.1.3 A compound which belongs to the same homologous series as compound B (1)
2.1.4 A plastic (1)
2.2 Write down the STRUCTURAL FORMULA of EACH of the following:
2.2.1 Compound C (3)
2.2.2 The acid used to prepare compound B (2)
2.2.3 The monomer used to make compound D (2)
2.3 Compound A reacts with an unknown reactant, X, to form 2-methylpropane.
Write down the:
2.3.1 NAME of reactant X (1)
2.3.2 Type of reaction that takes place (1)
[13]
