An experiment is conducted to investigate the relationship between the frequency of
light incident on a metal and the maximum kinetic energy of the emitted electrons from
the surface of the metal. This experiment is conducted for three different metals.
The graph below represents the results obtained.
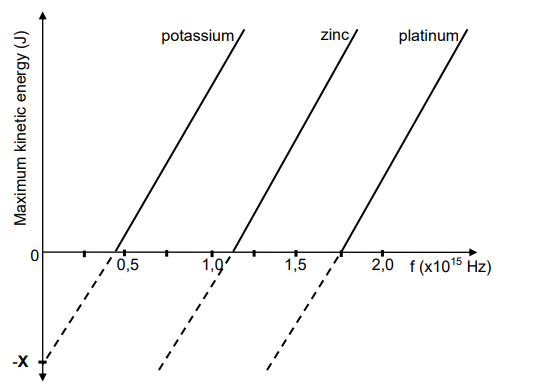
10.1 Name the phenomenon on which this experiment is based. (1)
10.2 Name the physical quantity represented by X on the graph. (1)
10.3 Which ONE of the three metals needs incident light with the largest
wavelength for the emission of electrons?
Give a reason for the answer. (2)
10.4 Define the term work function in words. (2)
10.5 Calculate the:
10.5.1 Work function of platinum (3)
10.5.2 Frequency of the incident light that will emit electrons from the
surface of platinum with a maximum velocity of 5,60 x 105 m∙s-1 . (4)
[13]
TOTAL: 150
