10.1 The experimental set-up below is used in a Grade 10 class to demonstrate
the electrical conductivity of a sodium chloride solution.
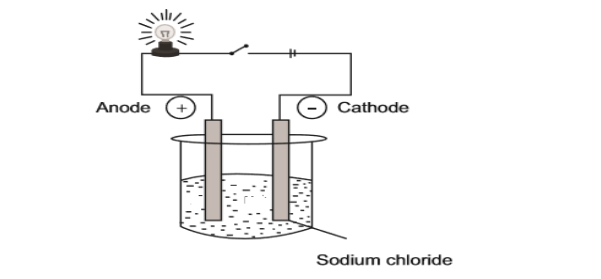
10.1.1 Define the term electrolyte. (2)
10.1.2 Write down the formula of ions present in this set-up. (1)
10.1.3 What will happen to the brightness of the bulb in the set-up above
if sodium chloride is replaced by calcium chloride?
Write down INCREASE, DECREASE or REMAIN THE SAME.
Give a reason for your answer. (3)
10.2 Study the reactions A and B below and answer the following questions.
A: 2Mg(s) + O2(g) → 2MgO(s)
B: 2NaI(aq) + Cℓ2(g) → 2NaCℓ(aq) + I2(g)
10.2.1 Which of the reactions is an ion exchange reaction? Give a reason
for your answer. (2)
10.2.2 Which of the reactions is a redox reaction? Give a reason for your
answer. (2)
10.3 A group of Grade 10 learners want to investigate the reaction of ions in
solutions. They pour the following solutions into six different test-tubes
marked A to F.

However, the learners forgot to write down which solution is in which test-tube.
10.3.1 Write down an equation to show how solid calcium carbonate
dissociates in water. (2)
10.3.2 Write down the name of the chemical they would use to test for
the presence of halides. (1)
10.3.3 How can they distinguish between the THREE types of halides
used during the test mentioned in QUESTION 10.3.2? (3)
10.3.4 Learners add a few drops of barium nitrate solution to test-tubes B
and C and a white precipitate is formed in each of the test-tubes.
They then add nitric acid solution to both test tubes. In test tube B
the precipitate DISSOLVES, whilst in test-tube C the precipitate
REMAINS.
Write down the name of the compound that was tested in:
(a) Test-tube B (1)
(b) Test-tube C (1)
10.3.5 What type of reaction takes place between the precipitation in B and
the nitric acid? (1)
[19]
