QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose
the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in
the ANSWER BOOK, e.g. 1.11 E. Each question has only ONE correct answer.
1.1 Nitrogen gas is an example of a/an …
A element.
B compound.
C heterogeneous mixture.
D homogeneous mixture. (2)
1.2 The heating curve, not drawn to scale, of a compound is shown below.
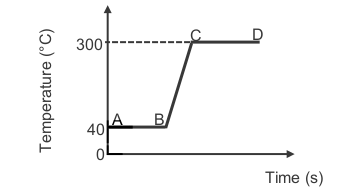
During which section(s) on the curve will the potential energy of the
compound INCREASE?
A BC only
B CD only
C AB and CD
D AB, BC and CD (2)
1.3 Elements in the periodic table are arranged in order of increasing …
A mass number.
B number of protons.
C number of neutrons.
D number of nucleons. (2)
1.4 Avogadro’s number is equal to the number of …
A atoms in 1 mole CO.
B atoms in 1 mole Br2 .
C molecules in 1 mole Au.
D molecules in 1 mole N2 . (2)
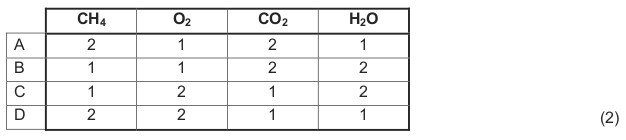
1.5 The unbalanced equation for a chemical reaction is shown below.
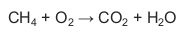
Which ONE of the following represents the coefficients of reactants and
products in the BALANCED equation?
1.6 A covalent bond forms …
A between metal and non-metal atoms.
B through electron transfer.
C through sharing of electrons.
D between positive and negative ions. (2)
1.7 The reaction between hydrogen chloride (HCℓ) and sodium hydroxide (NaOH)
is an example of a/an … reaction.
A redox
B acid-base
C precipitation
D gas forming (2)
1.8 Consider the Aufbau diagram of an element below.
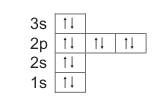
The element is a/an …
A halogen.
B noble gas.
C alkali metal.
D alkaline-earth metal. (2)
1.9 Which ONE of the following equations represents a precipitation reaction?
A NaOH + HCℓ → NaCℓ + H2O
B NaCℓ + HNO3 → NaNO3 + HCℓ
C NaCℓ + AgNO3 → AgCℓ + NaNO3
D Na2 CO3 + 2HCℓ → 2NaCℓ + CO2 + H2O (2)
1.10 The air surrounding the Earth is the …
A biosphere.
B lithosphere.
C atmosphere.
D hydrosphere. (2)
[20]
