Four options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 Which ONE of the following is a good conductor of electricity?
A Wood
B Plastic
C Silicon
D Copper (2)
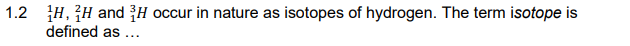
A the group and the period number of an element in the periodic table.
B an atom of the same element having the same number of protons but a different number of neutrons.
C the mass of a particle on a scale where an atom of carbon-12 has a mass of 12.
D the most probable region in space where electrons have the specific
energy corresponding to the orbitals. (2)
1.3 Refer to the table below to fill in the spaces in the following sentence
The density of the metals … and that of non-metals … across period 2 of the periodic table

1.4 Which ONE of the following molecular mass represents hydrogen chloride?
A 57,5
B 35,5
C 36,5
D 40,2 (2)
1.5 The bonding between crystal lattices can only be a/an … bond.
A ionic
B metallic
C covalent
D pure (2)
1.6 Study the diagram below.
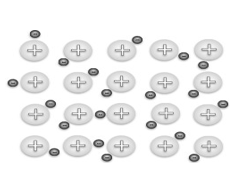
The diagram above represents the following:
A A lattice structure between ions.
B A buckminsterfullerene of sulphur
C Intermolecular forces between molecules
D Positive metal kernels and the sea of delocalised electrons (2)
1.7 Thando slowly heats a chocolate bar and observes the changes. Which ONE of the following is the most accurate conclusion?
A Chocolate undergoes a chemical change
B Chocolate undergoes a physical change
C Chocolate decomposes into its constituent components
D Chocolate undergoes no change (2)
1.8 Study the equation below:
2H2(g) + O2(g) > 2H2O(g)
A 2 molecules of hydrogen gas react with 1 atom of oxygen gas to form 2 atoms of water vapour
B 2 moles of hydrogen gas react with 1 mole of oxygen gas to form 2 moles of water vapour
C 4 atoms of hydrogen gas react with 2 molecules of oxygen gas to form 2 moles of water vapour.
D 4 g of hydrogen gas react with 16 g of oxygen gas to form 18 g of water vapour.
(2)
1.9 An aqueous mixture in a test tube contains Ag+(aq), K+(aq) and Pb+(aq). How
many different solids will form when NaCℓ(aq) is added to this mixture?
A None
B 1
C 2
D 3 (2)
1.10 SO2 gas dissolves in water and contributes to acid rain. What is the name of the acid that forms during this reaction?
A Sulfuric acid
B Carbon dioxide acid
C H2CO3
D Carbonic acid (2)
[20]
