QUESTION 3 (Start on a new page.)
3.1 The three isotopes of magnesium are Mg-24, Mg-25 and Mg-26. The
percentage abundance of the three isotopes is 80%, 10% and 10%
respectively.
3.1.1 Define the term isotope. (2)
3.1.2 Calculate the relative atomic mass of magnesium. (4)
3.1.3 The number of protons and electrons, the mass number and the
atomic number of Mg-24 and its ion are shown in the table below.
Some of these values in the table have been omitted. Write down
the letters (a–e) in the ANSWER BOOK and next to each letter the
number omitted.

3.2 The sp notation of an unknown element X is shown below.
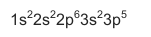
For element X, write down the:
3.2.1 Number of valence electrons (1)
3.2.2 Period where this element is found on the periodic table (1)
3.2.3 Highest energy level in which electrons occur (1)
3.2.4 Symbol (1)
Magnesium combines with element X to form a compound.
3.2.5 Write down the type of bond that forms between magnesium and
element X. (1)
3.2.6 Draw the Aufbau diagram for the MAGNESIUM ION. (2)
3.2.7 Draw Lewis dot diagrams to show the bond formation between
magnesium and element X. (4)
[22]
