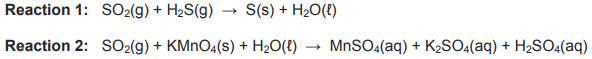The unbalanced equations for two redox reactions, in which SO2 is involved, are shown below.
9.1 Explain what is meant by the term redox reaction. (2)
9.2 Write down the oxidation number of Mn in:
9.2.1 KMnO4 (1)
9.2.2 MnSO4 (1)
9.3 Is Mn in Reaction 2 OXIDISED or REDUCED? Give a reason for the answer. (2)
9.4 In which reaction, Reaction 1 or Reaction 2, does SO2 act as an oxidising agent? Give a reason for the answer. (2)
9.5 Write down the oxidation half-reaction in Reaction 1. (2)
9.6 Use the Table of Standard Reduction Potentials and write down the balanced net ionic equation for Reaction 1. Show the half-reactions and how you arrived at the final equation. (4)
[14]