QUESTION 3 (Start on a new page.)
The boiling points of five organic compounds (P, Q, R, S and T) are studied.
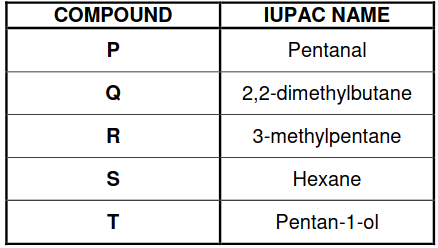
3.1 Define the term boiling point. (2)
The boiling points of compounds Q, R and S are compared.
3.2 Give a reason why this is a fair comparison. (1)
The boiling points of Q, R and S are given below (NOT necessarily in the correct
order).
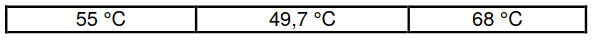
3.3 Which ONE of the three boiling points is most likely the boiling point of
compound R? Explain the answer. (4)
3.4 A mixture of equal amounts of P and T is placed in a flask and heated to a
temperature below their boiling points. Assume that no reaction or
condensation takes place. The vapour produced is collected in a syringe.

3.4.1 Which compound (P or T) will be present in a greater amount in the
SYRINGE? (2)
3.4.2 Explain the answer to QUESTION 3.4.1 by referring to the TYPES
and STRENGTHS of intermolecular forces. (3)
[12]
