The electrochemical cell illustrated below is set up under standard conditions
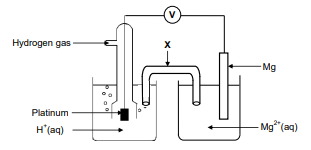
8.1 Component X completes the circuit in the cell. State ONE other function of
component X. (1)
8.2 Define the term anode. (2)
8.3 Identify the anode in the cell above. (1)
8.4 Write down the:
8.4.1 Reduction half-reaction that takes place in this cell (2)
8.4.2 NAME or FORMULA of the reducing agent in this cell (1)
8.5 Calculate the initial voltmeter reading of this cell under standard conditions. (4)
8.6 The Mg|Mg2+ half-cell is now replaced by a Cu|Cu2+ half-cell. It is found that
the direction of electron flow changes.
Fully explain why there is a change in direction of electron flow by referring to
the relative strengths of the reducing agents involved. (3)
[14]
