The diagrams below show two electrochemical cells in which carbon electrodes are used. In cell A, concentrated copper (II) chloride solution is used and in cell B, liquid aluminium oxide is used.
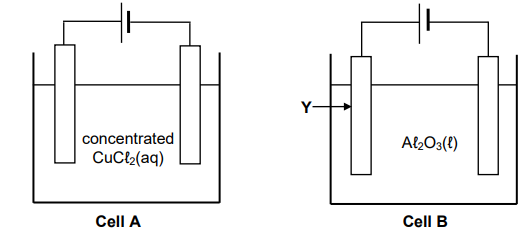
9.1 What type of electrochemical cell, ELECTROLYTIC or GALVANIC, is shown
above? Give a reason for the answer. (2)
9.2 Write down the:
9.2.1 Half-reaction that takes place at the anode of cell A (2)
9.2.2 Half-reaction that takes place at the cathode of cell B (2)
9.2.3 NAME or FORMULA of the product formed at the cathode of cell A (1)
9.3 Give a reason why the mass of electrode Y decreases after a while. (1)
[8]
