8.1 When a piece of sodium metal (Na) is added to water in a test tube, hydrogen gas is released. When phenolphthalein indicator is added to the test tube, the solution turns pink.
8.1.1 Define the term reduction in terms of electron transfer. (2)
8.1.2 Write down the reduction half-reaction. (2)
8.1.3 Write down the balanced equation for the reaction that takes place (3)
8.1.4 Give a reason why the solution turns pink. (1)
When a piece of copper is added to water in a test tube, no reaction is observed.
8.1.5 Refer to the relative strengths of the REDUCING AGENTS to explain why no reaction is observed. (3)
8.2 Consider the cell notation below.
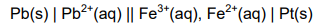
8.2.1 What does the single line (|) in the cell notation above represent? (1)
8.2.2 State the energy conversion that takes place in this cell. (1)
8.2.3 Calculate the initial emf of the cell under standard conditions. (4)
[17]
