MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions.
Each question has only ONE correct answer. Choose the answer and write only
the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK,
for example 1.11 E.
1.1 The type of bond formed between a H+ ion and H2O is called a/an …
A hydrogen bond.
B dative covalent bond.
C ionic bond.
D covalent bond. (2)
1.2 The shape of the molecule in which the central atom is surrounded by two
lone pairs and two bonding pairs is …
A linear.
B trigonal planar.
C tetrahedral
D bent. (2)
1.3 The intermolecular forces in dry ice (CO2) are …
A ion-induced dipole forces.
B hydrogen bonding.
C ion-dipole forces.
D London forces. (2)
1.4 The bond energy of a C–Cℓ bond is 338 kJ.mol-1 whereas the bond energy of
a C–I bond is 238 kJ.mol-1. The difference in bond energy exists because …
A the bond length of the C–Cℓ bond is greater than that of the C–I bond.
B chlorine is more electronegative than iodine.
C the bond length of the C–I bond is greater than that of the C–Cℓ bond.
D the chlorine atom is bigger than the iodine atom. (2)
1.5 A gas of volume V is at a temperature T1 and pressure P1 in a gas syringe.
If the pressure on the gas is doubled and the temperature halved, then the
volume that the gas will occupy is …
A 1⁄4 V
B 1⁄2 V
C V
D 2 V (2)
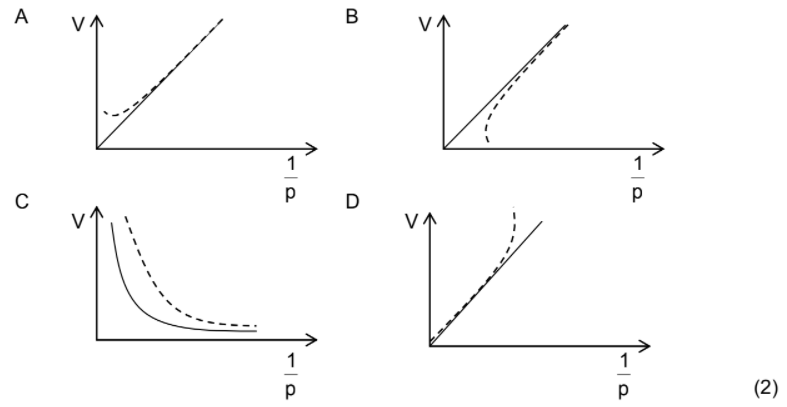
1.6 Which ONE of the graphs below CORRECTLY represents the deviation of
a real gas from ideal gas behaviour at very high pressures? The dotted line
represents the graph of the real gas.
1.7 The flowers of hydrangeas are natural indicators of soil pH. A natural indicator
is made in a laboratory by using hydrangea flowers. NaOH and HCℓ are
added to the indicator and the colour change is recorded in the table below.

If orange juice is added to the indicator above, the observed colour may be …
A brown.
B pink.
C purple.
D blue. (2)
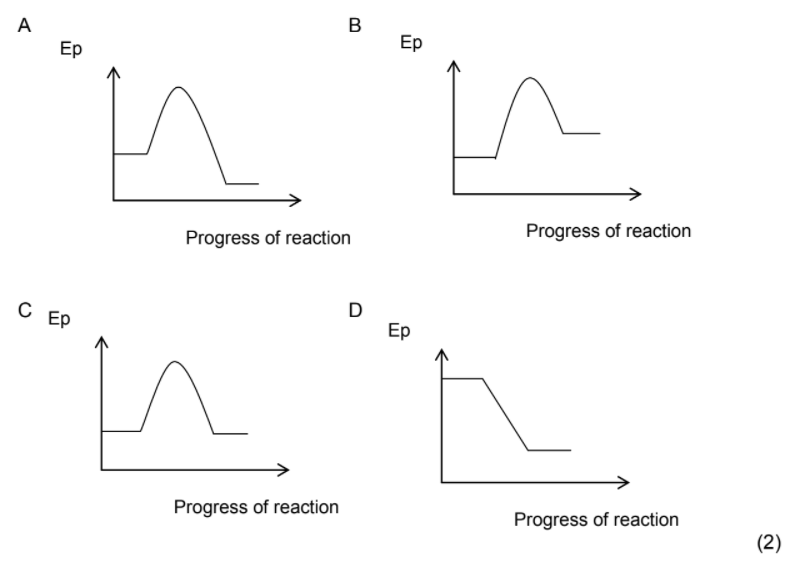
1.8 Cellular respiration occurs inside the cells of all living organisms.
Oxygen reacts with glucose in cellular respiration to produce the following
compounds according to the balanced equation below:
C6H12O6(aq) + 6O2 (g) → 6CO2(g) + 6H2O(ℓ) ΔH = -2 830 kJ
The potential energy versus progress of reaction diagram for this reaction
is …
1.9 The oxidation number of phosphorus in H3PO4 is …
A +3
B -2
C +2
D +5 (2)
1.10 During the processing of gold ore, zinc is added to the gold cyanide solution
to produce gold according to the balanced equation below:
Zn(s) + 2NaAu(CN)2(aq) → 2Au(s)+ Zn(CN)2(aq) + 2NaCN(aq)
The reducing agent in this reaction is …
A Na+
B Au+
C Zn
D CN– (2)
[20]
