QUESTION 8 (Start on a new page.)
A standard electrochemical cell is set up using two standard half-cells, as shown in the
diagram below.
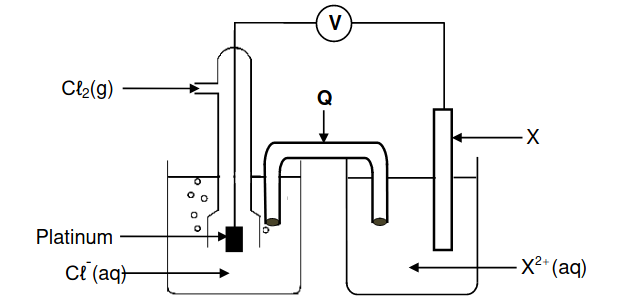
8.1 State the energy conversion that takes place in this cell. (1)
8.2 What is the function of component Q? (1)
X is a metal. A voltmeter connected across the cell initially registers 1,49 V.
8.3 Use a calculation to identify metal X. (5)
8.4 Write down the NAME or FORMULA of the reducing agent. (1)
8.5 The reading on the voltmeter becomes ZERO after this cell operates for
several hours.
8.5.1 Give a reason for this reading by referring to the rates of oxidation
and reduction half-reactions taking place in the cell. (1)
A silver nitrate solution, AgNO3(aq), is NOW added to the chlorine half-cell
and a precipitate forms.
8.5.2 How will the reading on the voltmeter be affected?
(Choose from INCREASES, DECREASES or REMAINS the same) (1)
8.5.3 Use Le Chatelier’s principle to explain the answer to
QUESTION 8.5.2. (2)
[12]
