7.1 Ethanoic acid (CH3COOH) is an ingredient of household vinegar
7.1.1 Is ethanoic acid a WEAK acid or a STRONG acid? Give a reason for
the answer. (2)
7.1.2 An ethanoic acid solution has a pH of 3,85 at 25 °C. Calculate the
concentration of the hydronium ions, H3O+(aq), in the solution. (3)
Sodium ethanoate, CH3COONa(aq), forms when ethanoic acid reacts with sodium hydroxide.
7.1.3 Will the pH of a sodium ethanoate solution be GREATER THAN 7,
LESS THAN 7 or EQUAL TO 7? (1)
7.1.4 Explain the answer to QUESTION 7.1.3 with the aid of a balanced
chemical equation. (3)
7.2 Household vinegar contains 4,52% ethanoic acid, CH3COOH by volume
A 1,2 g impure sample of calcium carbonate (CaCO3) is added to 25 cm3
household vinegar
On completion of the reaction, the EXCESS ethanoic acid in the household vinegar is neutralised by 14,5 cm3 of a sodium hydroxide solution of concentration 1 mol∙dm-3. The balanced equation for the reaction is:
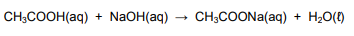
7.2.1 Calculate the number of moles of the unreacted ethanoic acid. (3)
7.2.2 Calcium carbonate reacts with ethanoic acid according to the
following balanced equation:

Calculate the percentage calcium carbonate in the impure sample if 1 cm 3
of household vinegar has a mass of 1 g. (8)
[20]
