QUESTION 7 (Start on a new page.)
7.1 The relationship between conductivity and the concentration of ions in two
electrolytes, NaCℓ(aq) and CaCℓ 2 (aq), of the SAME concentration are
investigated.
7.1.1 Define the term electrolyte. (2)
7.1.2 Is the water molecule POLAR or NON-POLAR? Give a reason for
the answer. (2)
7.1.3 For this investigation, write down the:
(a) Independent variable (1)
(b) Dependant variable (1)
The NaCℓ(aq) is added dropwise to distilled water in a beaker and the
conductivity of the solution is measured after the addition of each drop. The
experiment is repeated for the CaCℓ2 (aq). The results obtained are shown in
the table below.

7.1.4 Write down a balanced equation for the dissociation of NaCℓ(s) in
water. (3)
7.1.5 Which electrolyte, NaCℓ(aq) or CaCℓ2 (aq), has the higher
conductivity? Give a reason for the answer. (2)
7.2 A learner is supplied with two unlabelled bottles containing potassium salts.
She knows that one bottle contains a SULPHATE and the other a
CARBONATE.
To distinguish between the two salts she adds a few drops of barium nitrate,
Ba(NO3)2 (aq), to a solution of each salt in two separate test tubes, A and B,
as shown below.
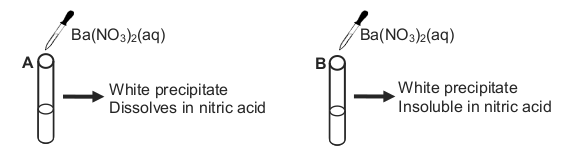
The learner finds that a white precipitate forms in each test tube. After the
addition of nitric acid, the precipitate in test tube A dissolves to release a gas,
while the precipitate in test tube B remains.
Write down the:
7.2.1 FORMULA of the POTASSIUM SALT in test tube A (2)
7.2.2 FORMULA of the precipitate that forms in test tube B (2)
7.2.3 Balanced equation for the reaction that explains why the precipitate
dissolves in test tube A after the addition of nitric acid (3)
[18]
