The dissociation of iodine molecules to iodine atoms (I) is a reversible reaction taking
place in a sealed container at 727 °C. The balanced equation for the reaction is
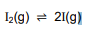
Kc for the reaction at 727 °C is 3,76 x 10-3
6.1 Write down the meaning of the term reversible reaction (1)
6.2 At equilibrium the pressure of the system is increased by decreasing the
volume of the container at constant temperature.
How will EACH of the following be affected? Choose from INCREASES,
DECREASES or REMAINS THE SAME.
6.2.1 The value of the equilibrium constant (1)
6.2.2 The number of I2 molecules (1)
6.3 Explain the answer to QUESTION 6.2.2 by referring to Le Chatelier’s
principle. (2)
6.4 At 227 °C, the KC value for the reaction above is 5,6 x 10-12.
Is the forward reaction ENDOTHERMIC or EXOTHERMIC?
Fully explain the answer. (4)
6.5 A certain mass of iodine molecules (I2) is sealed in a 12,3 dm3 flask at a temperature of 727 °C (Kc = 3,76 x 10-3).
When equilibrium is reached, the concentration of the iodine atoms is found to be 4,79 x 10-3 mol∙dm-3. Calculate the INITIAL MASS of the iodine molecules
in the flask. (9)
[18]
.
