QUESTION 6 (Start on a new page.)
Initially 60,8 g pure carbon dioxide, CO2(g), is reacted with carbon, C(s), in a sealed
container of volume 3 dm3. The reaction reaches equilibrium at temperature T
according to the following balanced equation:
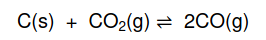
6.1 Define the term chemical equilibrium. (2)
6.2 At equilibrium it is found that the concentration of the carbon dioxide is
0,054 mol∙dm-3.
Calculate the:
6.2.1 Equilibrium constant, KC, for this reaction at temperature T (7)
6.2.2 Minimum mass of C(s) that must be present in the container to
obtain this equilibrium (3)
6.3 How will EACH of the following changes affect the AMOUNT of CO(g) at
equilibrium?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
6.3.1 More carbon is added to the container (1)
6.3.2 The pressure is increased by reducing the volume of the container
at constant temperature.
Use Le Chatelier’s principle to explain the answer. (3)
6.4 The table below shows the percentages of CO2(g) and CO(g) in the container
at different temperatures.
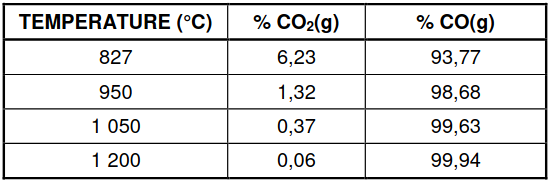
6.4.1 Is the reaction EXOTHERMIC or ENDOTHERMIC?
Refer to the data in the table and explain the answer. (3)
6.4.2 Use the information in the table to determine temperature T.
Show clearly how you arrived at the answer. (3)
[22]
