MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Write down the question number (1.1–1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE:

1.1 In a chemical reaction an oxidising agent will …
A lose protons.
B gain protons.
C lose electrons.
D gain electrons. (2)
1.2 A catalyst is added to a reaction mixture at equilibrium.
Which ONE of the following statements about the effect of the catalyst is FALSE?
A The rate of the forward reaction increases.
B The rate of the reverse reaction increases.
C The equilibrium position shifts to the right.
D The equilibrium position remains unchanged. (2)
1.3 What product will be formed when an alkene reacts with water vapour (H2O) in the presence of an acid catalyst?
A Ester
B Alkane
C Alcohol
D Aldehyde (2)
1.4 Which ONE of the following represents a SUBSTITUTION REACTION?
A CH2 = CH2 + HBr → CH3CH2Br
B CH2 = CH2 + H2O → CH3CH2OH
C CH3CH2OH → CH2 = CH2 + H2O
D CH3CH2OH + HBr → CH3CH2Br + H2O (2)
1.5 Consider the two organic molecules I and II below.
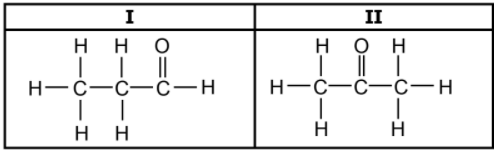
Which ONE of the following represents the homologous series to which
compound I and compound II belong? (2)

1.6 Consider the balanced equations for three reactions represented below:
I: N2(g) + 3H2(g) ⇌ 2NH3(g)
II: 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g)
III: 2NO(g) + O2(g) ⇌ 2NO2(g)
Which of the above reactions form(s) part of the Ostwald process?
A I only
B II only
C III only
D II and III only (2)
1.7 Which ONE of the following pairs is NOT a conjugate acid-base pair?
A H3O+ and OH−
B NH+4 and NH3
C H2PO–4 and HPO2-4
D H2CO3 and − HCO–3 (2)
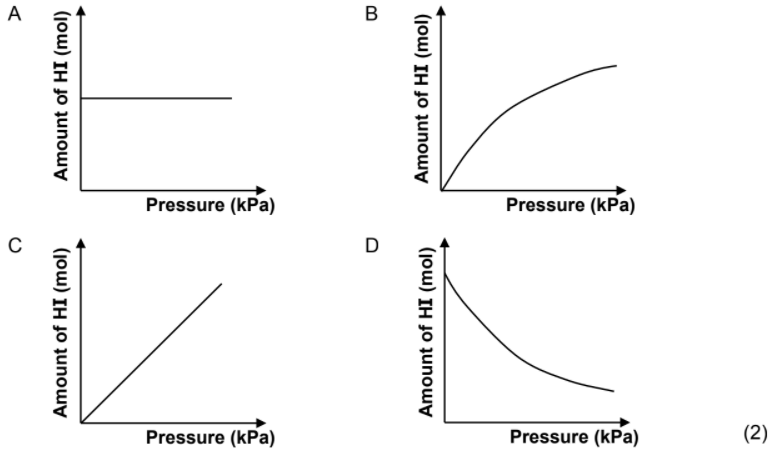
1.8 The reaction between hydrogen gas and iodine gas reaches equilibrium in a closed container according to the following balanced equation:
H2(g) + I2(g) ⇌ 2HI(g)
Which ONE of the graphs below shows the relationship between the amount of HI(g) at equilibrium and the pressure in the container at constant temperature?
1.9 Which ONE of the equations below represents the half-reaction occurring at the CATHODE of an electrochemical cell that is used to electroplate an object?
A Ag → Ag+ + e
B Cr3+ + 3e– → Cr
D Cr3+ + e– Cr2+
C Cu2+ +e– → Cu+ (2)
1.10 Equal amounts of magnesium (Mg) powder react respectively with equal volumes and equal concentrations of HCl(aq) and H2SO4(aq), as shown below.

The magnesium is in EXCESS.
Consider the following statements regarding these two reactions:
I: The initial rate of the reaction in test tube X equals the initial rate of the reaction in test tube Y.
II: After completion of the reactions, the mass of magnesium that remains in test tube X will be greater than that in test tube Y.
III: The amount of hydrogen gas formed in X is equal to the amount of hydrogen gas formed in Y.
Which of the above statements is/are TRUE?
A I only
B II only
C III only
D I and III only (2)
[20]
