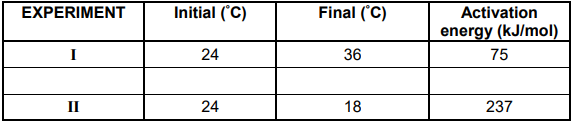
Learners study ENDOTHERMIC and EXOTHERMIC reactions by conducting experiments I and II in which the reactions shown in the table below take place.

The learners measured the initial and final temperatures of the reaction mixtures.
They also obtained activation energies for the reactions from a data table.
The learners represented their findings in a table as shown below
5.1 Define the term activation energy. (2)
5.2 In which experiment (I or II) is the reaction EXOTHERMIC?
Explain your answer. (2)
5.3 Is the heat of the reaction, ΔH, POSITIVE or NEGATIVE for an
EXOTHERMIC reaction? (1)
5.4 Write down the general name of a substance that can be added to the
reaction mixture in experiment II to reduce the activation energy. (1)
5.5 Both reactions produce the same number of moles of oxygen gas.
How does the mass of H2O2 used in experiment I compare to the mass of
H2O used in experiment II?
Write down only SMALLER THAN, LARGER THAN or THE SAME. (2)
5.6 Draw a potential energy versus time graph for the reaction in experiment II.
The following must be shown on the graph.
- Heat of the reaction (ΔH)
- Activation energy (Ea) (3)
[11]
