Grade 11 learners want to verify the relationship between temperature and volume of a gas. They used the following experimental set-up.

4.1 Write down the name of the gas law that is investigated. (1)
4.2 For this investigation write down the:
4.2.1 Investigative question (2)
4.2.2 Controlled variable (1)
4.3 Write down the name of apparatus X. (1)
4.4 The learners plot the results of their investigation on the graph below:
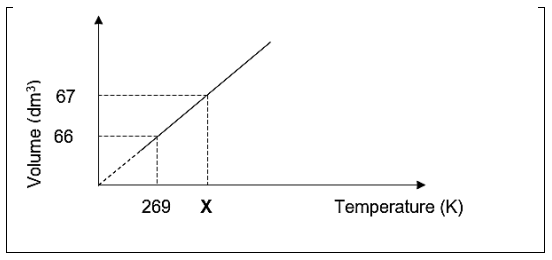
4.4.1 Determine, by calculation, the value of X.
132 g of CO2 gas was used in the above investigation. (4)
4.4.2 Calculate the pressure of the gas at 269 K. (5)
4.5 Write down the TWO conditions of temperature and pressure at which real
gases deviate from the ideal gas behaviour (2)
4.6 The CO2 used in the investigation is replaced with an equal amount of
H2 (g).
Which gas (CO2 or H2) behaves more closely to an ideal gas?
Give TWO reasons for your answer. (3)
[19]
