Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A−D), next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 The distance between the nuclei of two adjacent atoms when atoms bond is called …
A bond length.
B bond energy.
C interatomic bond.
D intermolecular forces. (2)
1.2 Which ONE of the following substances has ION-DIPOLE forces?
A H2O (ℓ)
B CO2 (g)
C NaCℓ (aq)
D NaCℓ (s) (2)
1.3 The geometrical shape of the PCℓ5 molecule according to VSEPR theory is …
A linear.
B trigonal planar.
C tetrahedral.
D trigonal bipyramidal. (2)
1.4 ONE mole of water (H2O) and ONE mole of carbon dioxide (CO2) will have the same…
A mass.
B molar mass.
C number of molecules.
D density. (2)
1.5 A certain mass of oxygen is sealed in a syringe. The gas exerts a pressure p. If both the volume and the temperature are doubled, the new pressure of the gas will be …

1.6 The relationship between pressure and volume of a fixed amount of gas at constant temperature is BEST described by …
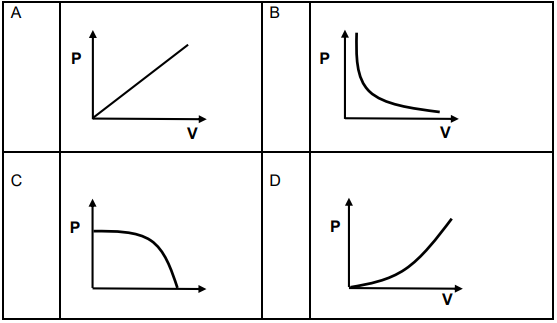
1.7 Equal masses of each of the following gases He, O2, CH4 and N2 are placed in separate containers at the same temperature and pressure.
Which ONE of the gases will have the LARGEST volume?
A He
B O2
C CH4
D N2 (2)
1.8 Consider the reaction:

X represents …
A H2O acting as an acid.
B H2O acting as a base.
C H3O+ acting as an acid.
D H3O+ acting as a base. (2)
1.9 Consider the pairs of reactants given below.
Which ONE of the following pairs of reactants will produce a salt, water and carbon dioxide?
A Zn + H2SO4
B NaOH + HCℓ
C CuO + H2SO4
D Na2CO3 + HCℓ (2)
1.10 Consider the following redox reaction:
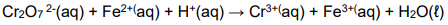
The product of the reduction half reaction in the equation is …
A Fe3+
B Cr3+
C H2O
D H+ (2)
[20]
