QUESTION 4 (Start on a new page.)
A section of the periodic table is shown below. Imaginary symbols are used to
represent some of the elements.
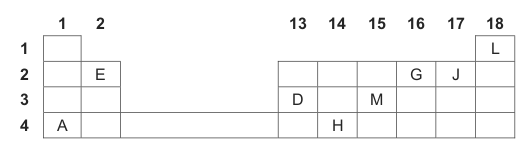
4.1 Write down the IMAGINARY SYMBOL, as shown above, of the element that:
4.1.1 Is a halogen (1)
4.1.2 Will form a cation with a charge of +2 (1)
4.1.3 Has the largest atomic radius (1)
4.1.4 Has the highest electronegativity (1)
4.1.5 Is a metalloid (1)
4.1.6 Is a noble gas (1)
4.1.7 Will form a diatomic molecule (1)
4.1.8 Has three valence electrons (1)
4.2 The first ionisation energy of element A is 400 kJ·mol -1 .
4.2.1 Define the term first ionisation energy. (2)
4.2.2 The first ionisation energy of element A can be represented by the
following incomplete equation:

Copy the equation above into the ANSWER BOOK and complete it. (2)
4.3 Atoms of element J release the most energy when gaining electrons to form
negative ions.
Write down ONE word or term for the underlined phrase. (1)
4.4 Write down the formula of the compound formed when:
4.4.1 D combines with G (2)
4.4.2 A combines with J (2)
[17]
