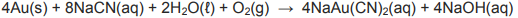The balanced chemical equation for the EXTRACTION of gold from its ore is as follows:
10.1 State ONE disadvantage of using cyanide (CN– ) in the extraction of gold. (1)
10.2 Will the final solution of the extraction process be ACIDIC or BASIC (ALKALINE)? Give a reason for the answer. (2)
10.3 Determine the oxidation number of gold in NaAu(CN)2. (1)
10.4 Write down the FORMULA of the reducing agent in the reaction above. (1)
Zinc powder is now used to PRECIPITATE the gold.
The balanced equation for the reaction is:
10.5 Does zinc undergo OXIDATION or REDUCTION during the precipitation reaction? (1)
10.6 Write down a half-reaction to support the answer to QUESTION 10.5. (2)
10.7 Calculate the percentage of gold in NaAu(CN)2. (2)
[10]
TOTAL: 150