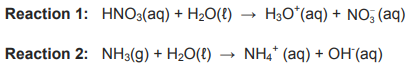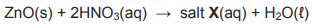8.1 Consider the balanced equations for the reaction of water with nitric acid and ammonia below
8.1.1 Define an acid in terms of the Lowry-Brønsted theory. (2)
8.1.2 Write down the FORMULA of ONE conjugate acid-base pair in Reaction 1. (2)
8.1.3 Is the solution formed in Reaction 1 ACIDIC or BASIC (ALKALINE)? Give a reason for the answer. (2)
8.1.4 Define the term ampholyte. (2)
8.1.5 Write down the FORMULA of a substance that acts as an ampholyte in the reactions above. (1)
8.1.6 Explain the answer by referring to the role of this substance in Reaction 1 and Reaction 2. (2)
100 cm3 of HNO3 of a concentration of 0,2 mol·dm-3 is diluted to 0,16 mol·dm-3.
8.1.7 Calculate the volume of water that must be added to the 0,2 mol·dm– 3 HNO3. (4)
8.2 Zinc oxide, ZnO, is insoluble in water and can be harmful to the environment. Nitric acid can be used to neutralise zinc oxide.
The incomplete equation for the reaction is:
8.2.1 Calculate the mass of zinc oxide that can be neutralised by 80 cm3 of nitric acid with a concentration of 0,16 mol·dm-3. (5)
8.2.2 Write the NAME and FORMULA of salt X that forms during this reaction. (2)
[22]