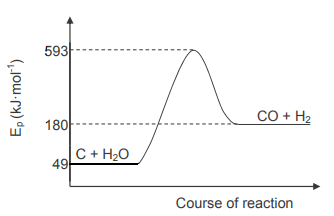
The balanced equation for the reaction of carbon with steam is as follows:
C(s) + H2O(g) → CO(g) + H2(g)
The graph below, NOT drawn to scale, represents the change in potential energy of
the substances during the reaction.
7.1 Define the term heat of reaction. (2)
7.2 Is the reaction ENDOTHERMIC or EXOTHERMIC? Give a reason for the answer. (2)
7.3 Use the information on the graph and write down the value of the:
7.3.1 Activation energy (2)
7.3.2 Heat of reaction (2)
[8]
