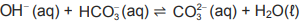Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 E. Each question has only ONE correct answer.
1.1 The number of valence electrons in a silicon atom is …
A 4
B 6
C 14
D 28 (2)
1.2 In a polar covalent bond …
A the difference in electronegativity between two atoms is zero.
B electrons are shared unequally between two atoms
C electrons are transferred from the less electronegative atom to the more electronegative atom.
D delocalised electrons are shared between atoms. (2)
1.3 The type of intermolecular forces present between N2 molecules are …
A triple bonds.
B dipole-dipole forces.
C hydrogen bonds.
D London forces. (2)
1.4 Which ONE of the following contains ionic bonds?
A OF2
B H2O
C CH3Cℓ
D NaCℓ (2)
1.5 The number of ions present in 3 moles of MgCℓ2 is …
A 3 × 6,02 × 1023
B 6 × 6,02 × 1023
C 9 × 6,02 ×1023
D 12 × 6,02 × 1023 (2)
1.6 Two different gases of the same volume at STP will have the same …
A mass.
B density.
C molar mass.
D number of molecules. (2)
1.7 4 moles of nitrogen gas is sealed in a balloon at temperature T and pressure p. The volume of the balloon changes from V to 2V when the temperature is increased to 1,5T.
The new pressure in the balloon is …
A 0,75p
B 1,33p
C 1,5p
D 3p (2)
1.8 Consider the chemical equation below:
The Lowry-Brønsted bases in the above reaction are …
A HCO3 (aq) and OH– (aq)
B H2O(ℓ) and OH– (aq)
C H2O(ℓ) and ![]()
D OH– (aq) and ![]() (aq) (2)
(aq) (2)
1.9 A few drops of bromothymol blue indicator are added to a hydrochloric acid
solution, HCℓ(aq). When ammonium hydroxide, NH4OH(aq), is added to this
solution, the colour of the indicator will change from …
A blue to yellow.
B yellow to blue.
C yellow to red.
D blue to red. (2)
1.10 Oxidation takes place when the …
A reducing agent loses electrons.
B oxidising agent loses electrons.
C reducing agent gains electrons.
D oxidising agent gains electrons. (2)
[20]